April 2025
GENSPEED in the “Kronen Zeitung”, Austria’s most popular newspaper!
Our novel test for detection of prosthetic joint infections mentioned in the article is currently being evaluated at Charité in Berlin!
March 2025

GENSPEED at the APOkongress in Schladming
New evidence of GENSPEED‘s unique capabilities ! 

In our latest publication in Bone and Joint Research conducted in collaboration with the esteemed team at Charité – Universitätsmedizin Berlin – we showcase how our rapid multiplex micro-ELISA can simultaneously measure alpha-defensin, calprotectin, and IL-6 in a single, 20-minute test using just microliters of synovial fluid.
This three-in-one assay has the potential to be a future game changer for diagnosing periprosthetic joint infection (PJI), providing faster, more precise results at the point of care.
What makes it exceptional:
• Unique Multiplexing of three biomarkers in one test
• Fast Turnaround for more immediate treatment decisions
• Low Detection Limits – well below established PJI thresholds
• Patient-Centered: Earlier, more accurate diagnosis can reduce hospital stays and improve outcomes
We’re excited for the broader impact this technology can have, once clinically validated, on patients worldwide.
Read the full open-access article here: https://boneandjoint.org.uk/Article/10.1302/2046-3758.143.BJR-2024-0100.R1
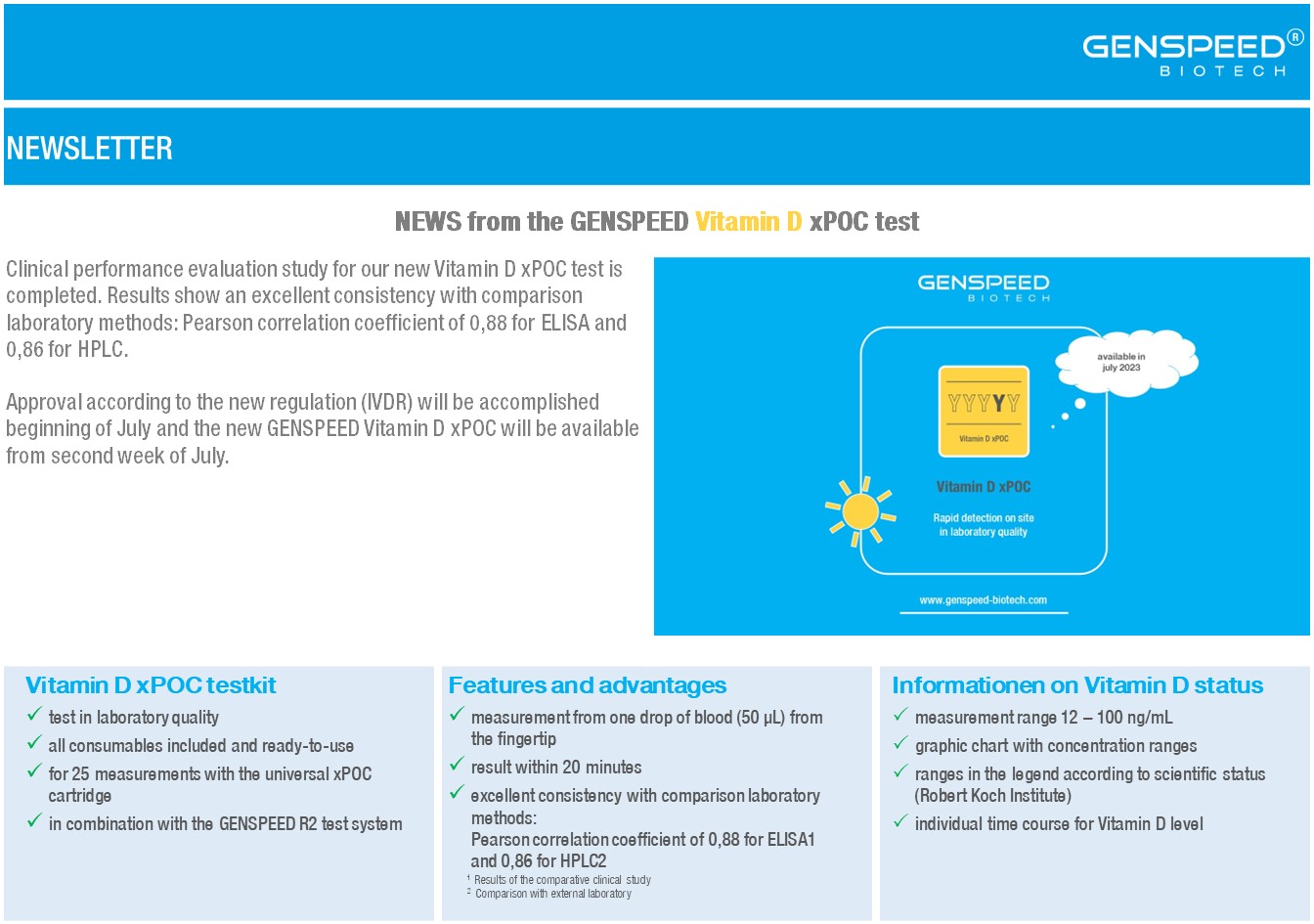
NEWS from the Vitamin D xPOC test
The suPARnostic POC+
Great news suPARnostic POC+ Test based on GENSPEED technology now available!
GENSPEED Biotech GmbH, CEO, Max Sonnleitner says: “We are excited to see the commercial launch of suPARnostic® POC+ and the positive clinical data from the Greek study, which demonstrates the ability to run suPAR analysis on GENSPEED devices near patients with reliable results that can be obtained in just 20 minutes out of a drop of blood. This is an important milestone in our collaboration with ViroGates, and we look forward to continuing our work together to incorporate additional relevant biomarkers in multiplex tests in the future”
Original post: https://lnkd.in/dUcf6pmr

COMPAMED 14.-17. November 2022
We are at the COMPAMED in Düsseldorf -melting pot for high-tech solutions in the medical technology sector!
Even twice 😉
You can meet us in Hall 8A
- at the Microfluidics Innovation Hub (booth K32) and
- at MedPhab (booth # F35.1)


NEW! FSME/TBE IgG xPOC antibody test

Low-entry-barrier point-of-care testing of anti-SARS-CoV-2 IgG in the population of Upper Austria from December 2020 until April 2021 - a feasible surveillance startegy for post-pandemic monitoring?
Abstract: …The aim of this study was to estimate the prevalence of SARS-CoV-2-specific immunoglobulin G antibodies in the federal state of Upper Austria (Austria) during the period of December 2020 until April 2021. To achieve this goal, we have analyzed anonymized data on the immune status of self-referral volunteers that have been determined at local pharmacies through a low-entry-barrier point-of-care analysis approach. The seroprevalence values for immunoglobulin type G antibodies against SARS-CoV-2 antigens obtained by rapid diagnostic testing on peripheral blood from volunteers reflect the current population-based estimates reported in the literature as well as the positivity rates detected by PCR-screening analyses. In conclusion, broad-based monitoring of IgG antibodies by means of a point-of-care testing network represents a valuable tool to assess the current immune situation within regionally defined populations.



